Abstract
Background: Primary central nervous system lymphoma (PCNSL) is a brain tumor with a very poor prognosis. Tirabrutinib is a second-generation inhibitor of Bruton's tyrosine kinase with higher kinase selectivity than ibrutinib (Banerji et al., 2020), suggesting potentially fewer off-target adverse events-including infections, bleeding, and cardiovascular adverse events-that have been reported with ibrutinib. On the basis of results of a phase I/II study (ONO-4059-02; trial registration number: JapicCTI-173646), tirabrutinib was approved in Japan in March 2020 for recurrent or refractory PCNSL at a dose of 480 mg under fasted conditions. We conducted a prospective, noninterventional, multicenter, observational study (trial registration number: jRCT2011210002) to evaluate adverse drug reactions (ADRs) and the overall response rate (ORR) of tirabrutinib against recurrent or refractory PCNSL in Japan. This postmarketing all-case surveillance study started on May 20, 2020. We report the interim safety and effectiveness data for patients who started tirabrutinib administration from the start date through October 31, 2020.
Methods: All patients were monitored up to 52 weeks. Collected data included baseline characteristics, laboratory test results, ADRs, and the ORR. ADRs were coded according to MedDRA, version 24.1. The ORR was evaluated according to the criteria of the International PCNSL Collaborative Group (IPCG). The timing and method of imaging study was at discretion of physician.
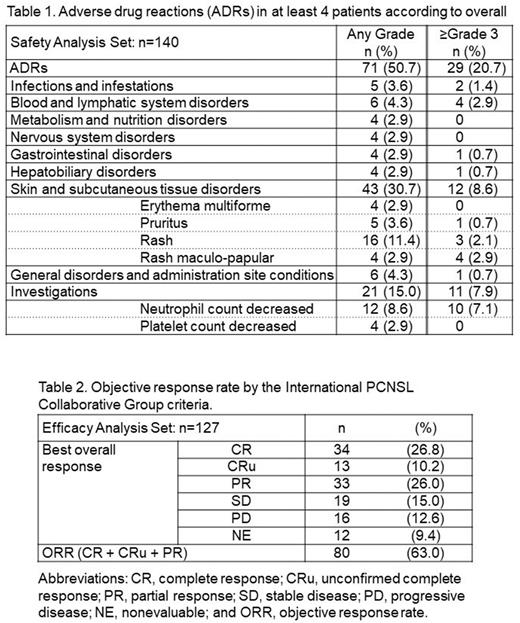
Results: Data from 140 patients who received tirabrutinib treatment were analyzed for safety, and data from 127 of those patients were analyzed for effectiveness. A total of 13 patients were excluded from the effectiveness analysis because 9 patients had a history of prior treatment with tirabrutinib and 4 patients received tirabrutinib as off-label use for the treatment of newly diagnosed disease. Of the 140 patients, 72 (51.4%) were male. Their median age was 70 years (range, 30-88 years), and 98 patients (70.0%) were 65 years of age or older. The Karnofsky Performance Status scores were 80-100 for 49 patients (35.0%), 50-70 for 60 patients (42.9%), and ≤40 for 31 patients (22.1%). Fifteen patients (10.7%) had intraocular lymphoma. The median number of previous lines of treatment was 1 (range, 0-6) and for 67 patients (47.9%), tirabrutinib was second-line therapy. The median duration of tirabrutinib treatment was 147.0 days (range, 3-365 days); 28 patients (20.0%) continued taking tirabrutinib until the end of the observation period (365 days) and thereafter. Of the 95 patients who discontinued tirabrutinib treatment, 60 (63.2%) did so because of disease progression, 15 (15.8%) because of adverse events, 13 (13.7%) because they transferred to another hospital, 4 (4.2%) because they responded to treatment, and 13 (13.7%) for other reasons. The most common systemic ADRs were skin and subcutaneous tissue disorders, including rash, pruritus, erythema multiforme, and rash maculo-papular; these affected 43 (30.7%) of all 140 patients, of whom 12 (8.6%) had grade 3 or worse ADRs (Table 1). Seven patients (5.0%) discontinued tirabrutinib treatment due to grade 3 or worse (n = 6) or grade 2 (n = 1) skin-related ADRs. Infections and infestations were observed in 5 patients (3.6%), of whom 2 (1.4%) had grade 3 or worse infections or infestations. Grade 1 or 2 bleeding was observed in 2 patients (1.4%). No patients in this cohort had cardiovascular ADRs or grade 5 ADRs. At the end of the 52 weeks, 73 patients (52.1%) were still alive, 46 (32.9%) had died, and the status of 21 (15.0%) was unknown. With regard to effectiveness (n = 127), the ORR was 63.0% (n = 80) and the rate of confirmed or unconfirmed complete response was 37.0% (n = 47; Table 2).
Conclusion: In this interim period of the postmarketing all-case surveillance study, the most common ADRs were skin and subcutaneous tissue disorders, and no new safety concerns were identified in addition to those in the clinical trial. The ORR and the rate of complete or unconfirmed complete response were comparable to those at the approved dose in the phase I/II study.
Disclosures
Kawasaki:ONO Pharmaceutical: Current Employment. Matsushita:ONO Pharmaceutical: Current Employment. Yoshida:ONO Pharmaceutical: Current Employment. Matsukura:ONO Pharmaceutical: Current Employment. Izutsu:Eizai, Chugai, Janssen, AstraZeneca, Novartis, Bristol Myers Squibb, Kyowa Kirin ,AbbVie, Ono Pharmaceutical , Eli Lilly, MSD, Daiichi Sankyo, Symbio, Takeda: Honoraria; Beigene, AstraZeneca, Ono Pharmaceutical, AbbVie, Novartis, Chugai: Consultancy; MSD, AstraZeneca, AbbVie, Eizai, Incyte, Janssen, Yakult, Kyowa Kirin, Ono Pharmaceutical, Daiichi Sankyo, Chugai, Beigene, Genmab, Loxo Oncology: Research Funding. Nishikawa:ONO Pharmaceutical: Consultancy; Chugai Pharm, Eisai, MSD: Research Funding; ONO Pharmaceutical: Honoraria; Varian Medical Systems, Nippon Kayaku: Speakers Bureau; Novocure: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.